Nearly 95% of rare diseases lack FDA-approved treatments. Why? Rare diseases rarely get trial opportunities. The key to new therapies lies in collecting the right data at the right time with the right tools.
Beyond the Diagnosis
Rare diseases affect 400 million globally.* Many patients face delayed diagnosis and limited treatments. Their journey is characterized by a constant search for adequate care and support, stressing the critical need for specialized research and patient-focused solutions to improve their quality of life.
We are dedicated to raising awareness and making a difference to the rare disease community today and every day, through focusing research efforts in this area of vast unmet medical need.
The conventional one-size-fits-all trial strategy often fails to capture the full spectrum of patient experiences. Resorting to primary endpoints as the only measures relevant to trial success doesn’t measure the outcomes that matter most to patients and their caregivers.
Emmes designs and provides the technology for personalized, validated assessments relevant to patients' daily lives—efficiently producing impactful results and outcomes that can be accepted by regulators and payers when designed with this purpose in mind.
*Source: https://globalgenes.org/rare-disease-facts/

Designing Fit-for-Purpose Endpoints
Rare disease trials require tailored solutions for endpoint selection and data analysis, prioritizing outcomes that matter most to patients and caregivers.
Discover new ways to customize assessments to complement the outcomes dictated by regulators in rare disease studies.
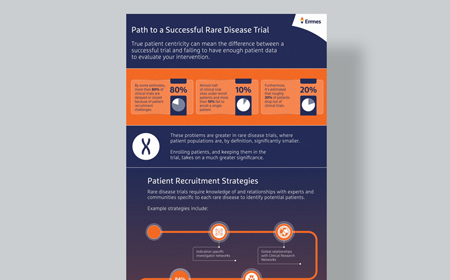
Unlock the Path to Trial Success
Gain insights into patient-centric strategies in rare disease trials.
See how the intersection of patient-centricity and innovation enhances patient recruitment and retention.
Why Emmes for Rare Disease Trials
Our team always stays close to the patient—partnering with advocates and caregivers to develop assessments, offering guidance from patients and families directly to sponsors, reducing trial burden by listening to the needs of patients in the trial setting and addressing obstacles to participation, and pioneering new ways to document disease progression and treatment benefits by developing innovative tools.
We engage with key opinion leaders and regulators to ensure that novel, patient-focused outcomes are recognized, paving the way for treatments that truly address patients’ needs.

Talk to Our Rare Disease Team
Discuss with our Emmes Experts how we can help you with your rare disease trials.






